Introduction: Chimeric antigen receptor (CAR) T-cell therapy fails to achieve durable responses in 60% of relapsed/refractory (R/R) large B-cell lymphoma (LBCL) patients in the third or later line setting. There is no standard treatment after CAR T-cell therapy progression and a wide range of outcomes are observed in this patient population. Besides the interval between CAR T-cell infusion and progressive disease (PD), data regarding prognostic factors at time of progression are scarce. Our aim was to develop a new prognostic tool to predict overall survival (OS) after CAR T-cell therapy progression with easily-available markers from routine clinical practice.
Methods: First, we performed a retrospective data collection at 12 Spanish centers of R/R LBCL patients who progressed after CAR T-cell therapy in the third or later line setting from September 2018 until June 2022 (training cohort, TC). We analyzed a total of 15 variables, including pre-CAR T-cell therapy characteristics (gender, histology, primary refractory disease, previous hematopoietic transplant, number of previous lines) and values collected at time of progression to CAR T-cells (age, stage, extranodal sites, ECOG, hemoglobin, neutrophils, platelets, LDH, best response to CAR T-cells, time from CAR T-cell infusion to PD). The primary endpoint was OS from date of progression to CAR T-cell therapy. A stratified Cox model was used to estimate hazard ratios (HRs) using post-progression treatment as a stratification factor. We used LASSO regression with minimum lambda to identify which variables had the highest prognostic impact on OS. Additionally, the factors with a lower contribution were eliminated to create a parsimonious model. The C-statistic was used to evaluate its discrimination. We examined the performance of the International Prognostic Index (IPI) score and Revised IPI (R-IPI) in this setting. Finally, we tested the score in an external validation cohort (VC) which included a comparable patient population from 3 European countries.
Results: Among the 216 LBCL patients included in the TC, most were male (66%), had an ECOG of 1 (48%) and stage IV disease (71%) at time of CAR T-cell therapy progression. Median time from CAR T-cell infusion to PD was 2.5 months (95CI% 1.9-2.9) and median follow-up from progression was 15 months. Salvage treatment was classified into 3 subgroups, including immunotherapy or targeted agents (43%), chemotherapy or radiotherapy (20%) and palliative care (38%).
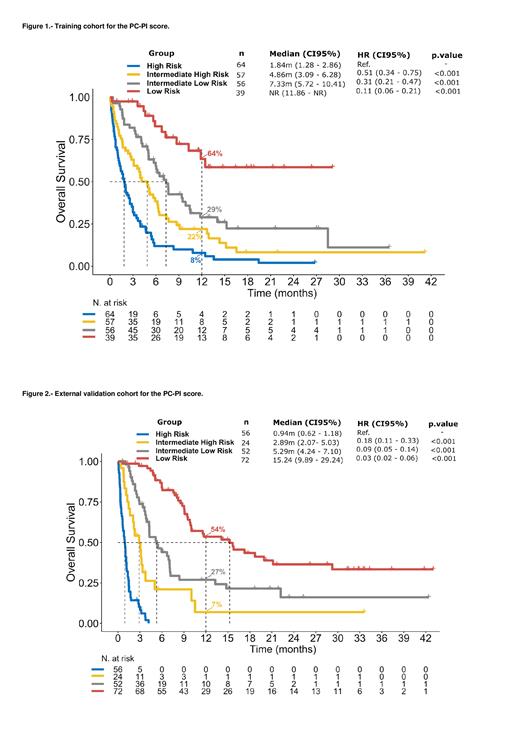
To build the prognostic score, a total of 5 variables were selected. Each marker received 1 point (given the similar HR [1.48-1.77]), if they met defined criteria: ECOG (>0), hemoglobin (<10 g/dL), LDH (>2 x upper normal limit), number of extranodal sites (>1) and time from CAR T-cell infusion to PD (<4 months). Patients with 0-1 points were classified as low risk, 2 points as intermediate-low risk, 3 points as intermediate-high risk and 4 or 5 points as high risk.
In the TC, the 4 risk groups showed statistically significant differences in OS (Figure 1). In the low-risk group (n=39 [18%]), the median OS [mOS] was not reached; in the intermediate-low risk (n=56 [26%]) mOS was 7.3 months (HR=2.89, p=0.002); in the intermediate-high risk (n=57 [26%]) mOS was 4.9 months (HR=4.81, p<0.001) and in the high risk (n=64 [30%]) mOS was 1.8 months (HR=6.69, p<0.001). In terms of post-relapse therapies, both the chemo/radiotherapy and the immunotherapy groups showed a balanced patient distribution, from low to high risk (32%, 35%, 14% and 19% vs 22%, 32%, 30% and 16%, respectively).
The VC included 204 patients with a similar patient distribution in the 4 prognostic risk groups (35%, 25%, 12%, 27%). The mOS for each of these groups was 15.2, 5.3, 2.9 and 0.9 months, respectively. Each group had distinct OS outcomes when compared with all the other risk groups (p<0.05 for each comparison) (Figure 2).
Finally, our model presented a C-index of 0.712 for the TC and 0.811 for the VC, outperforming both the IPI (0.647 [TC] and 0.691 [VC]) and R-IPI (0.632 [TC] and 0.683 [VC]).
Conclusions: The Post-CAR Prognostic Index (PC-PI) is a clinically useful tool for OS prediction and risk-adapted treatment planning in LBCL patients progressing after CAR T-cell therapy. In addition, our results will help stratification in clinical trials which include patients with prior CAR T-cell therapy.
Disclosures
Iacoboni:Autolus: Consultancy; Abbvie: Honoraria; Gilead Sciences: Consultancy, Honoraria; MSD: Honoraria; Novartis: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Janssen: Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; AstraZeneca: Honoraria. Sesques:KITE/GILEAD , BMS, JANSSEN, NOVARTIS, CHUGAI: Consultancy. Rejeski:Kite/Gilead: Other: Travel Support, Research Funding; Novartis: Honoraria; BMS/CELGENE: Consultancy, Honoraria; Pierre-Fabre: Other: Travel Support. Bastos-Oreiro:Kite-Gilead: Honoraria, Other: travel. Guerreiro:Novartis: Honoraria, Other: travel; Kite-Gilead: Honoraria, Other: travel; MSD: Honoraria, Other: TRAVEL; Pierre Fabre: Honoraria, Other: travel; BMS: Honoraria, Other: travel support. Martinez-Cibrian:Kite: Honoraria, Other: Travel support. Ghesquieres:Gilead, Roche: Consultancy; Gilead, Roche, BMS, Abbvie: Honoraria. Barba:Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pierre-Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nektar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lopez Corral:Janssen: Honoraria, Other: travel support; Novartis: Honoraria, Other: travel support; Gilead Sciences: Honoraria, Other: travel support. Reguera:AMGEN: Speakers Bureau; KITE: Speakers Bureau; BMS: Speakers Bureau; Janssen: Consultancy, Speakers Bureau. Sureda Balari:MSD: Research Funding; Kite: Consultancy, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau. Martin Garcia-Sancho:Clinigen: Consultancy; Eusa Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead / Kite: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; ADC Therapeutics America: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Ideogen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd, BMS / Celgene, Kyowa Kirin, Novartis, Gilead / Kite, Incyte, Lilly, ADC Therapeutics America, Miltenyi, Ideogen, Abbvie, Sobi: Consultancy; F. Hoffmann-La Roche Ltd, BMS/Celgene, Janssen, Gilead/Kite, Takeda, Eusa Pharma, Abbvie: Honoraria; Kyowa Kirin: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Kwon:Jazz: Speakers Bureau; Pfizer: Speakers Bureau; Kite-Gilead: Consultancy, Speakers Bureau. Kuhnl:Novartis: Honoraria, Research Funding; Kite Gilead: Honoraria; BMS: Honoraria; Abbvie: Honoraria. Subklewe:Gilead/Kite: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Seagen: Research Funding; Roche: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding; AstraZeneca: Speakers Bureau; Pfizer: Consultancy, Honoraria, Other: Travel Support, Speakers Bureau; Ichnos Sciences: Consultancy, Honoraria; AvenCell: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte Biosciences: Consultancy, Honoraria; Molecular Partners: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; GSK: Speakers Bureau; LAWG: Speakers Bureau; Springer Healthcare: Speakers Bureau; AbbVie: Consultancy, Honoraria; Autolus: Consultancy, Honoraria; advesya (CanCell Therapeutics): Consultancy, Honoraria; Genmab US: Consultancy, Honoraria; Interius BioTherapeutics: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Orbital Therapeutics: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Scare: Consultancy, Honoraria. Bachy:Takeda: Honoraria; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Incyte: Honoraria; Novartis: Honoraria, Other: Personal Fees; Pfizer: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Amgen: Research Funding; Roche: Consultancy, Honoraria; Kite, a Gilead Company: Honoraria, Other: Personal Fees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal